Understanding the Relationship between Risk and Substances of Interest in Disposable Hygiene Products
At Bostik, we take Substances of Interest (SOIs) seriously. We work together with the disposable hygiene industry to create a unified response for addressing SOIs. At the same time, consumers, governments, the media, and other organisations ask questions about what is in absorbent products and why. This can include questions about adhesives used in the production of disposable hygiene articles for:
- Baby care
- Feminine care
- Adult incontinence
SOI regulation to protect human health
Various governmental agencies have created regulations designed to protect public health. In Europe, The European Commission created REACH regulation (Registration, Evaluation, Authorisation, and Restriction of Chemicals) which application is controlled by ECHA (European Chemicals Agency). Under REACH, chemical manufacturers or importers must register the chemicals used to produce their polymers. Downstream users, such as disposable hygiene adhesive suppliers and end-product manufacturers that use chemicals, are also requested to identify and manage the potential risks linked to those chemicals.
In order to manage risks properly, it is necessary to clearly define four important terms — hazard, presence, exposure and risk. Four key points to keep in mind when considering SOIs are:
- Hazard: indicates the negative effect(s) (to health or the environment) associated with the SOI. A toxicological reference value (threshold) can be determined below which the corresponding negative effect(s) does not occur
- Presence: Indicates a SOI is detected within a sample.
- Exposure: Indicates a SOI can be in contact with the user (by oral, dermal and/or inhalation route).
- Risk: Indicates the chance of negative effects caused by the SOI to occur, based on all 3 previous parameters.
SOI risk assessment explained
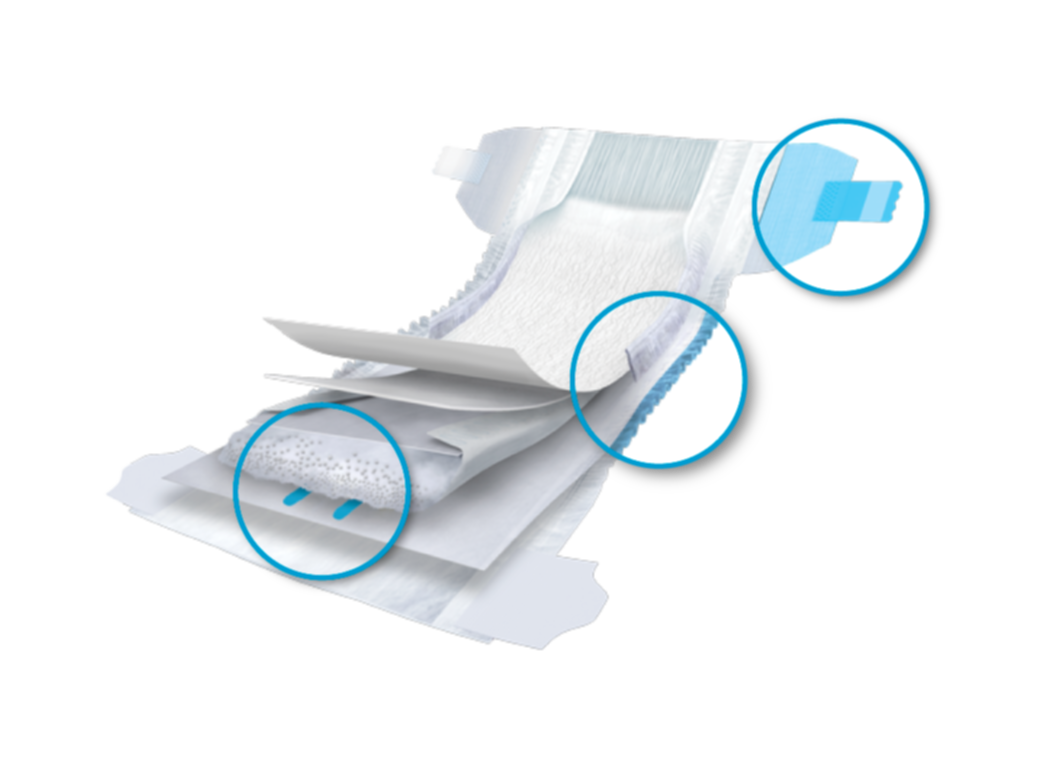
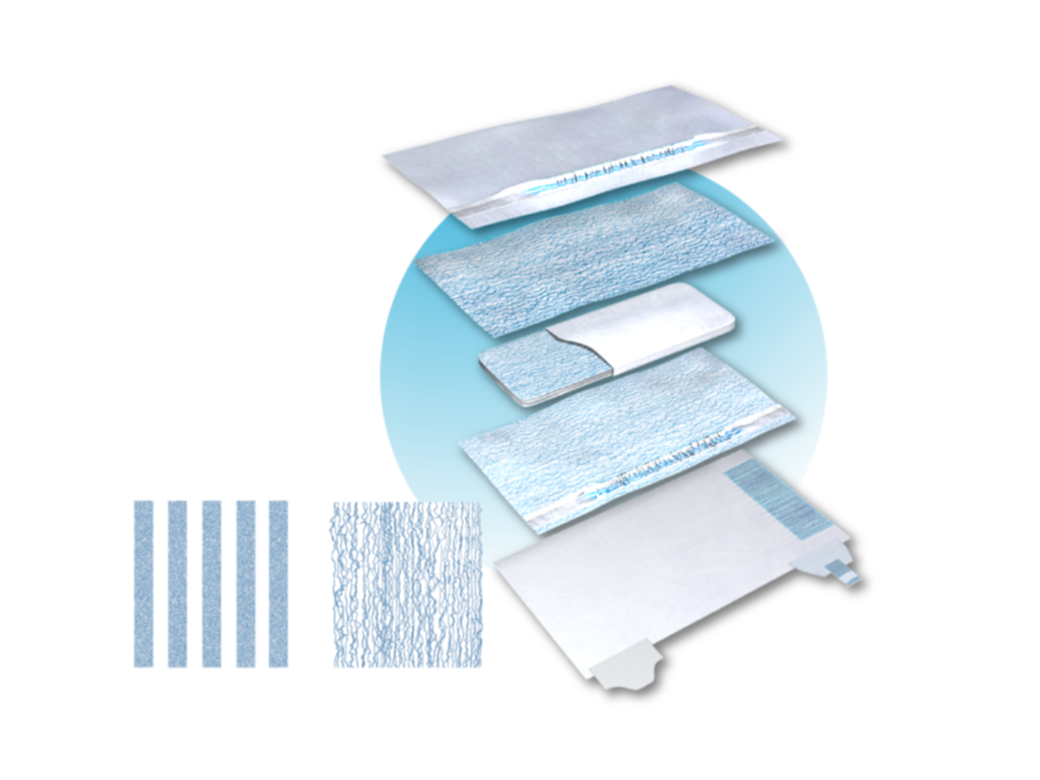
Once the hazard(s) of a substance are characterised by standardised studies conducted in independent laboratories, the substance’s presence and level are determined using accurate and specific testing protocols to extract the substance from the products, and specific analytical methods to measure the concentration of the substance. There are different ways to measure the presence of a substance. Some test protocols are quite harsh and are very far from how the product is actually used by consumers (i.e. submerging a product into solvents to determine SOI presence), some others are more designed to mimic the actual use of the product (i.e. exposing the product to synthetic urine and measuring SOI that transfers to the liquid). Therefore, alignment on the test protocol and analytical methods for substance presence is critical.
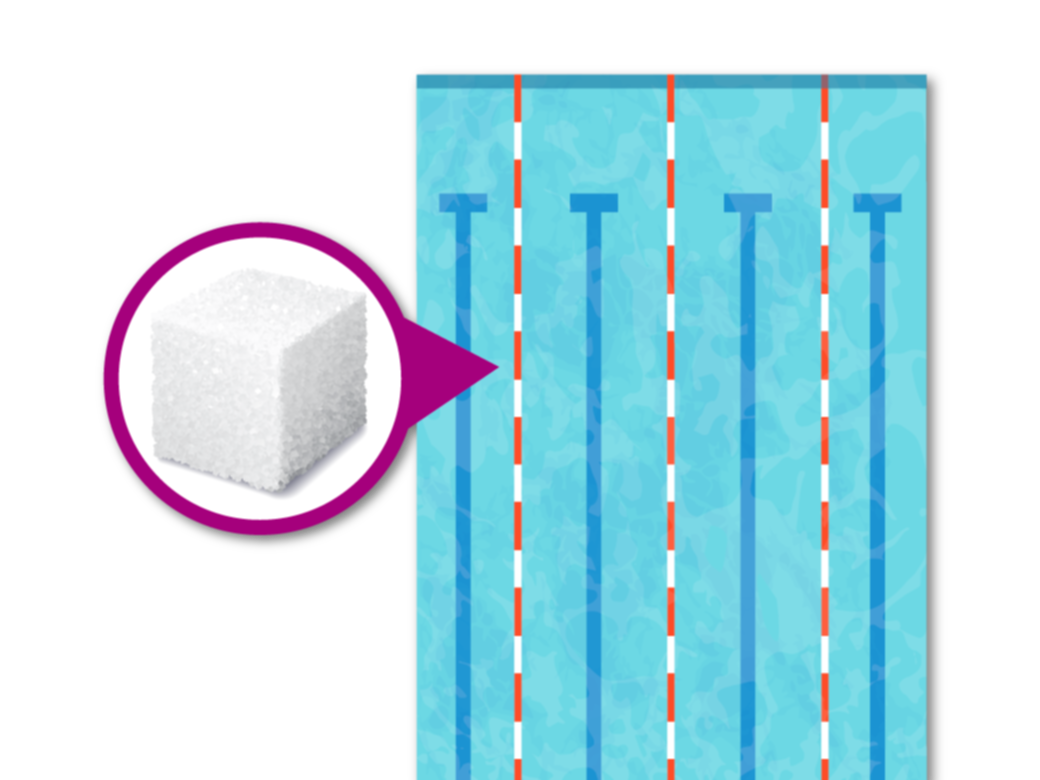
Once a standard test protocol and the analytical methods are chosen, it is necessary to understand the test’s degree of accuracy. This is especially true at the low concentrations often associated with SOIs, which may be given in parts per billion (ppb). To give it context, 2.4 ppb is the concentration of a single sugar cube dissolved in an Olympic-sized swimming pool. (We explore this analogy more thoroughly in our article, “Evaluating Presence, Exposure, and Risk.”)
Next, the product, as well as its use and handling, are evaluated to determine whether the user can be exposed to the substance and at what level. Here again, there are many approaches that can be used to determine exposure. Alignment on test methods is critical.
All this information, combined with the hazards associated with the substance itself, helps to evaluate the level of risk due to the substance under the conditions of use and determine if the risk is under control or not.
For example:
A harmful substance can be present at trace levels, but it may not be in contact with the user’s skin. If so, the exposure is insignificant and there is no risk of negative effects on health.
In another situation:
The substance can be in contact with the user’s skin, but the contact time and concentrations can be so small that the risk of negative effect on user’s health is insignificant.
Disposable hygiene products, health, and safety
Risk assessment allows governments and regulating authorities, as well as the disposable hygiene industry, to determine what level of presence of an SOI and conditions of use of the article are appropriate to have no risk for human health.
Defining and aligning on appropriate test methods is important for establishing safety guidelines. It is important that consistent methods are used for testing to ensure results are comparable to each other and to regulatory standards.
Efforts are being made to help consumers understand that potential presence of SOI does not necessarily mean exposure to this SOI. Similarly, exposure to SOI does not necessarily mean risk to human health. This may help the industry guide discussions in a productive and more transparent direction.
Partner with the experts at Bostik
When navigating the world of SOIs, you need a knowledgeable partner. At Bostik, our experts can make you aware of possible restrictions, offer support with how to deal with the regulatory process, and share how to make an assessment. Learn more.
Keep Reading
To find out more about SOIs, request a copy of our whitepaper about Substances of Interest in Disposable Hygiene.
CODE: SOI-21A04
SOI Materials from Bostik Academy
Click the links below to sign-in and access all of our Academy materials.